LogBB
Overview
This module provides mechanistic predictions of blood brain distribution of drugs and drug-like compounds in rodents. The predictive model is based on a data-set containing >500 brain to plasma partitioning ratios (expressed as LogBB constants) measured in mice and rats. Under the assumption of passive transport across blood-brain barrier LogBB is viewed as a cumulative effect of drug binding to plasma and brain constituents. Calculations therefore use octanol/water logP (main determinant of brain tissue binding) and unbound fraction in plasma (fu, plasma) as input parameters.
Features
- Calculates the extent of brain penetration (LogBB) and fraction unbound in brain tissue (fu,brain)
- Allows altering the values of main physicochemical determinants to get an insight on structural changes needed to achieve desired permeation characteristics
- Displays carrier-mediated transport alerts for compounds that undergo facilitated diffusion or active efflux across BBB
- Displays experimental LogBB values for up to three most similar compounds from the model training set along with literature references
Interface
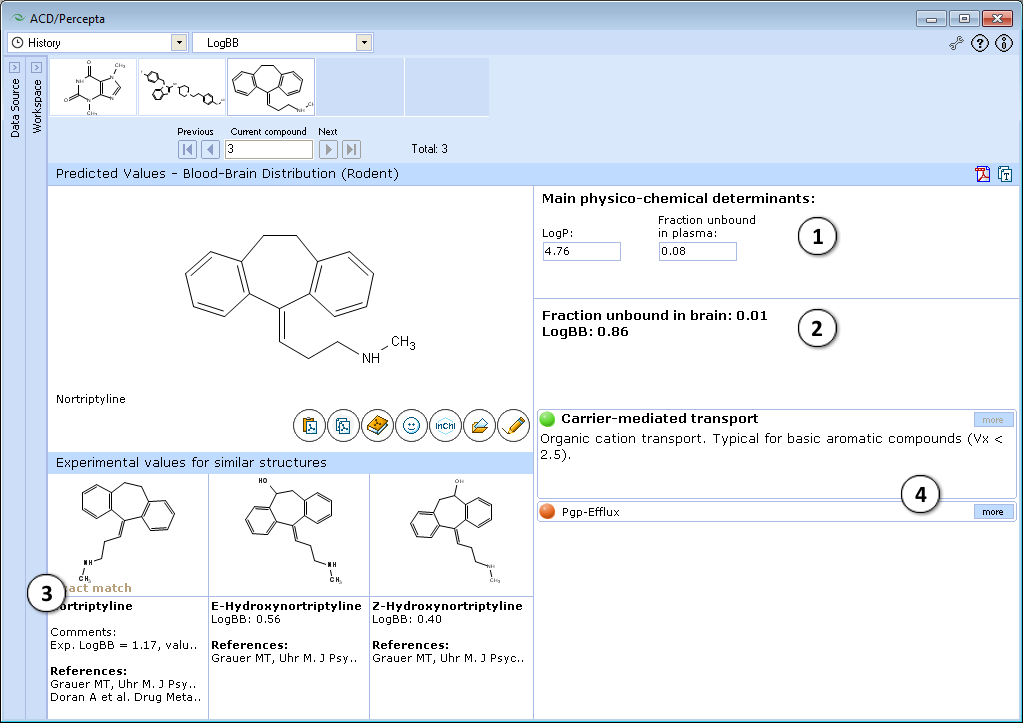
- logP and fraction unbound in plasma (fu, plasma) are calculated automatically. Alter these values to simulate the limiting effect of the compound's lipophilicity and/or plasma protein binding on its brain disposition.
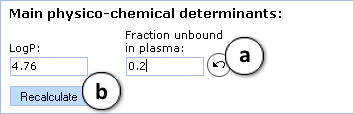
- a. Click the "Undo" button to restore the automatically calculated property value (fu, plasma in the current example) for a compound and recalculate LogBB using default parameter values
- b. Click to recalculate the unbound fraction in brain and LogBB using the currently specified parameter values
- Calculated values of unbound fraction in brain (fu, brain) and LogBB of a compound
- Up to 3 most similar structures from the training set of the model with experimental values and references
- An alert is shown if the compound is likely to exhibit other Blood-Brain Barrier transport mechanisms apart from passive diffusion: carrier-mediated influx or P-gp efflux
Technical information
Only a short summary of main technical aspects of BBB penetration predictors is given here. For a more detailed description of the modeling approach and underlying theory please refer to Lanevskij, K. et al. J Pharm Sci. 2009;98(1):122-34. [1]
Calculated quantitative parameters
BBB module in calculates the following quantitative characteristics of drug transport across blood-brain barrier:
Main parameters:
- Rate of brain penetration (Log PS). PS stands for Permeability-Surface area product and is defined from the kinetic equation of capillary transport (PS = -F * (1 - e-Kin/F)). By Its physical meaning PS is equal to the influx rate constant Kin corrected for blood flow rate in cerebral microcapillaries denoted as F.
- Extent of brain penetration (Log BB) determined by ratio of total drug concentrations in tissue and plasma at steady-state conditions (log BB = log(cbrainSS/cbloodSS).
Additional parameters:
- Fraction unbound in brain tissue (fu, brain)
- Brain/plasma equilibration rate given by log (PS * fu, brain) - combination of permeation rate and fraction unbound in brain.
When only single parameter values are considered fu, brain provides a better insight on drug disposition in CNS than Log BB since the former provides a direct estimate of tissue binding affinity/free (pharmacologically active) drug levels in brain, while the latter represents a superposition of compounds' binding to brain lipids and plasma proteins. However, both equilibrium distribution ratio (Log BB) and the rate of achieving this equilibrium (defined as log (PS * fu, brain) according to Liu X. et al. J Pharmacol Exp Ther. 2005;313(3):1254-62. [2]) must be evaluated to estimate whether enough compound permeates through BBB to reach pharmacologically active levels in brain.
Experimental data
The data sets used for modeling were compiled from original literature publications of BBB permeation studies in rodents. Quantitative log PS data were collected for more than 250 compounds. These were determined by one of the following experimental methods:
- Intravenous administration (IV)
- Brain uptake index (BUI)
- In situ brain perfusion.
Analysis of published brain/plasma distribution studies of drugs after intravenous (in some cases – intraperitoneal or oral) administration to rats and mice yielded a data set of log BB values for about 600 compounds. The collected data were thoroughly evaluated to ensure that they represent BBB transport by passive diffusion. The resulting data sets after preliminary analysis contained almost 200 log PS constants and almost 500 log BB ratios.
Modeling method & used descriptors
The predictive models of log PS and log BB constants were built using non-linear least squares regression. Non-linear fitting was necessary to account for the following aspects of the analyzed processes:
- Differentiation between diffusion-limited and cerebral blood flow-limited permeability (LogPS).
- Bilinear permeability - LogP dependence: rise of permeability with LogP up to a certain optimum point followed by subsequent decrease due to hydrophobic retention in the membrane (LogPS).
- Ionization-specific partitioning of electrolytes between phospolipid bilayer and plasma (LogPS) or brain tissue lipids and intesrtitial fluid (LogBB).
In both cases fitting was performed in a multi-step approach: the first stage involved determination of parameters describing membrane/plasma partitioning of neutral molecules while in the second stage stage these values wre fixed and eletrolyte-specific passive transport parameters were obtained.
Descriptors used for modeling included key physicochemical properties - octanol/water LogP of neutral species as a determinant of lipophilicity, ion form fractions at pH 7.4 calculated from the respective pKa values, number of hydrogen bond donors and acceptors in the molecule, and McGowan characetristic volume reflecting molecular size. All physicochemical property values were calculated with Algorithm Builder 1.8.
Prediction accuracy
Prior to development of both log PS and log BB predictive models a part of each data set was reserved for validation purposes and not used in modeling (internal validation set). Additionally, two independent data sets representing another type of experimental data (directly measured fu, brain values) were extracted from recent publications (Kalvass J. C. et al. Drug Metab Dispos. 2007;35(4):660-6 [3], and Summerfield S. G. et al. Xenobiotica. 2008;38(12):1518-35. [4]) These were used as external validation sets to confirm intrinsic correctness of the obtained model.
Performance of the models on various validation sets is presented in the table below:
| Data set | N | R2 | RMSE |
|---|---|---|---|
| log PS internal validation set | 53 | 0.82 | 0.49 |
| log fu, brain internal validation set | 137 | 0.74 | 0.41 |
| log fu, brain external validation set (Kalvass et al., 2007) | 31 | 0.73 | 0.42 |
| log fu, brain external validation set (Summerfield et al., 2008) | 20 | 0.70 | 0.36 |
Statistical parameters obtained for internal and external test sets demonstrate good predictive power of the models with RMSE being close to error of experimental determination in both cases.
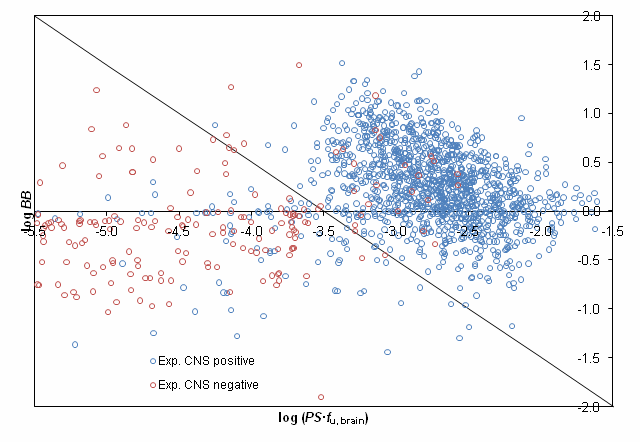
The proposed classification scheme was validated using experimentally assigned CNS activity categories for more than 1500 compounds presented in the study by Adenot M. & Lahana R. J Chem Inf Comput Sci. 2004;44(1):239-48. [5] After removing compounds, disposition of which is affected by P-gp efflux 92% of the remaining molecules were correctly classified by our model as shown in the figure below:
Transport mechanisms
The predictive models comprising the BBB module only account for passive transport of analyzed compounds across blood-brain barrier. In order to propose a clear physicochemical explanation of passive diffusion process, the data used for development of both LogPS and LogBB models were thoroughly evaluated to exclude values affected by enzymatic efflux or influx. For such compounds special alerts are displayed in all submodules related to the prediction of BBB transport parameters indicating that observed parameters for these molecules may be higher or lower than predicted values due to presence of carrier-mediated processes, such as facilitated diffusion or P-gp efflux. Considered influx transporters include:
- Amino-acid transport systems (System A, L, y+, x-)
- Glucose carrier GLUT1
- Organic cation/carnitine transporters (OCTN)
- Organic anion transporting polypeptide (OATP)
- Various other carriers specific for nucleotides and other endogenous substances.