Acute Toxicity Hazards: Difference between revisions
Created page with "==Overview== <br /> Acute Toxicity module performs rapid and accurate predictions of the tested compounds' LD50 values in rodents after various administration routes. The Acu..." |
No edit summary |
||
| Line 2: | Line 2: | ||
<br /> | <br /> | ||
The Acute Toxicity Hazards module is a knowledge-based expert system. It identifies hazardous fragments (toxicity alerts) that may be responsible for the high acute toxicity of compounds in rodents. | |||
<br /> | <br /> | ||
| Line 18: | Line 18: | ||
<br /> | <br /> | ||
# | # The highlighted part of molecule indicates the identified hazardous substructure that may be responsible for high acute toxicity of the compound. | ||
# | # Click to select the different administration route. | ||
# Up to 5 similar structures | # Identified hazardous substructures are presented in the form of tab list. Click the corresponding tab to select a hazardous fragment. The name and short description of hazardous fragments are described. Intensity of red ‘dot’ shows the difference between the mean LD50 value in the entire training set and its sub-set possessing a hazardous fragment. | ||
# Box-and-Whisher plot of acute toxicity in the training set and among compounds possessing the fragment. Median, 5%, 25%, 50%, 75%, 95% quantiles are presented. | |||
# Acute toxicity distribution density plot displays relationship between frequency of compounds possessing certain acute toxicity values in the entire training set and the LD50 values for the compounds, containing the highlighted hazardous fragment, indicated by red points. | |||
# Number of compounds, mean values and t-test for significance of difference between acute toxicity distribution density plots in the entire training set and the sub-set of compounds possessing hazardous fragment. | |||
# Up to 5 most similar structures in training set possessing the hazardous fragment with structures names, experimental values of LD50 (mg/kg) and similarity index. This index is based on QSAR analysis of logLD50 data.<br /> | |||
<br /> | <br /> | ||
| Line 73: | Line 37: | ||
A standard measure of acute toxicity is LD50 (lethal dose 50) defined as a dose that is lethal to 50% of the treated animals. It can be viewed as a “cumulative potential” to cause various acute effects and death of animals. To obtain a linear relationship with structural properties these data were converted to logarithmic form (pLD50) for modeling, but the final prediction results returned to the user are converted back to LD50 value (mg/kg). | A standard measure of acute toxicity is LD50 (lethal dose 50) defined as a dose that is lethal to 50% of the treated animals. It can be viewed as a “cumulative potential” to cause various acute effects and death of animals. To obtain a linear relationship with structural properties these data were converted to logarithmic form (pLD50) for modeling, but the final prediction results returned to the user are converted back to LD50 value (mg/kg). | ||
In | In ACD/Percepta LD50 is calculated for two different rodent species: toxicity for mice is estimated under oral, intraperitoneal, intravenous, subcutaneous administration, and for rats - under oral and intraperitoneal administration. | ||
===Model Features & Prediction Accuracy=== | ===Model Features & Prediction Accuracy=== | ||
Revision as of 09:49, 15 May 2012
Overview
The Acute Toxicity Hazards module is a knowledge-based expert system. It identifies hazardous fragments (toxicity alerts) that may be responsible for the high acute toxicity of compounds in rodents.
Features
- ACD/Percepta contains 86 “hazardous fragments”. These fragments were identified by analysis of toxicological literature and tested on acute toxicity data of more than 100,000 compounds.
- The toxicity distribution plots show significance of the identified hazardous fragments on the acute toxicity.
- Experimental LD50 value and similarity to test compound is shown for 5 most similar structures from the training set.
Interface
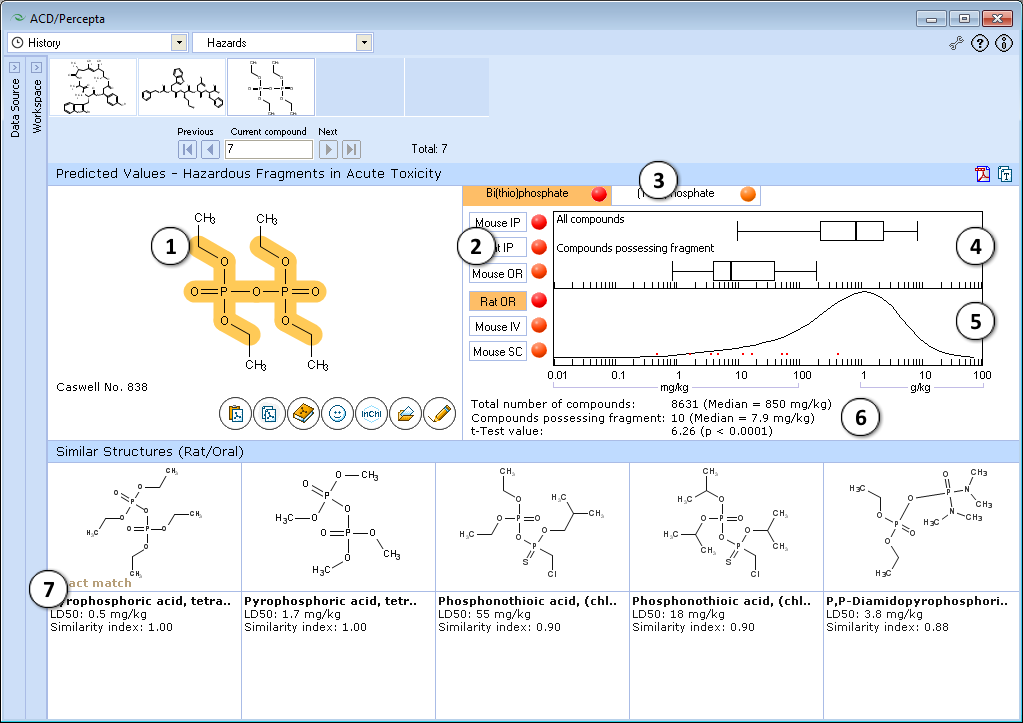
- The highlighted part of molecule indicates the identified hazardous substructure that may be responsible for high acute toxicity of the compound.
- Click to select the different administration route.
- Identified hazardous substructures are presented in the form of tab list. Click the corresponding tab to select a hazardous fragment. The name and short description of hazardous fragments are described. Intensity of red ‘dot’ shows the difference between the mean LD50 value in the entire training set and its sub-set possessing a hazardous fragment.
- Box-and-Whisher plot of acute toxicity in the training set and among compounds possessing the fragment. Median, 5%, 25%, 50%, 75%, 95% quantiles are presented.
- Acute toxicity distribution density plot displays relationship between frequency of compounds possessing certain acute toxicity values in the entire training set and the LD50 values for the compounds, containing the highlighted hazardous fragment, indicated by red points.
- Number of compounds, mean values and t-test for significance of difference between acute toxicity distribution density plots in the entire training set and the sub-set of compounds possessing hazardous fragment.
- Up to 5 most similar structures in training set possessing the hazardous fragment with structures names, experimental values of LD50 (mg/kg) and similarity index. This index is based on QSAR analysis of logLD50 data.
Technical information
Calculated quantitative parameter
A standard measure of acute toxicity is LD50 (lethal dose 50) defined as a dose that is lethal to 50% of the treated animals. It can be viewed as a “cumulative potential” to cause various acute effects and death of animals. To obtain a linear relationship with structural properties these data were converted to logarithmic form (pLD50) for modeling, but the final prediction results returned to the user are converted back to LD50 value (mg/kg).
In ACD/Percepta LD50 is calculated for two different rodent species: toxicity for mice is estimated under oral, intraperitoneal, intravenous, subcutaneous administration, and for rats - under oral and intraperitoneal administration.
Model Features & Prediction Accuracy
Acute Toxicity predictor was built using critically evaluated experimental data for more than 100,000 compounds extracted from RTECS and ESIS databases.
Fragmental QSAR (quantitative structure-activity relationship) was used for the development of baseline LD50 prediction model. The predictions are corrected according to the analysis of experimental LD50 values for similar compounds. Predictions are supported by reliability estimation that takes into account:
- Similarity of tested compound to the training set
- Difference between predicted LD50 for tested compound and experimental values for similar compounds
- Consistence of experimental values for similar compounds.
Predictive models of LD50 in various systems have been validated on external data sets. Validation results show that the accuracy of prediction is proportional to the reliability index. The predictions with a high or moderate reliability index are accurate (average prediction error is about 0.5-0.7 log units). A high/moderate reliability index was reported for 20-50% of compounds in the validation sets.
OECD Ranges
Chemicals are assigned to one of the five Oral Acute Toxicity Hazard Categories according to the numeric criteria expressed as LD50 (oral administration to rats). Categories were defined by OECD (Organization for Economic Cooperation and Development. A Guide to The Globally Harmonized System of Classification and Labeling of Chemicals (GHS)):
- V – LD50 2000-5000 mg/kg (may be harmful if swallowed)
- IV – 300-2000 mg/kg (harmful if swallowed)
- III – 50-300 mg/kg (toxic if swallowed)
- II – 5-50 mg/kg (fatal if swallowed)
- I < 5 mg/kg (fatal if swallowed).
Toxicity hazards
Toxicity Hazards is a knowledge-based expert system that identifies and highlights structural elements that may be responsible high acute toxicity of compounds in rodents. Tox Hazards system contains a list of 86 predefined 'toxicophores' compiled after thorough analysis of toxicological literature. Significance of the defined hazardous fragments was verified on acute toxicity data set (more than 100,000 compounds).