CYP2C19 Regioselectivity: Difference between revisions
Created page with "==Overview== <br /> The information about possible metabolites of a drug candidate is desirable in the earliest stages of drug development, but the experimental testing of bi..." |
m Fixed broken links |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 17: | Line 17: | ||
<br /> | <br /> | ||
[[Image: | [[Image:Cyp2c19_regioselectivity.png|center]] | ||
<br /> | <br /> | ||
| Line 31: | Line 31: | ||
<div class="mw-collapsible | <div class="mw-collapsible"> | ||
==Technical information== | ==Technical information== | ||
| Line 39: | Line 39: | ||
===Predicted endpoints=== | ===Predicted endpoints=== | ||
Cytochrome P450 Specificity-related modules in ACD/Percepta provide the following quantitative predictions: | |||
# '''P450 Substrates''': Probability that the compound of interest will be metabolized by a certain | # '''P450 Substrates''': Probability that the compound of interest will be metabolized by a certain cytochrome P450 isoform. | ||
# '''P450 Inhibitors''': Probability that the compound of interest will inhibit a certain | # '''P450 Inhibitors''': Probability that the compound of interest will inhibit a certain cytochrome P450 enzyme with IC50 below defined threshold. Two types of predictive models utilizing different IC50 thresholds have been developed. "General inhibition" models estimate whether the analyzed compound will exhibit any clinically significant cytochrome P450 inhibition at all (IC50 < 50 μM), while "Efficient inhibition" models predict probability that the compound will inhibit selected enzyme with IC50 < 10 μM. | ||
# '''P450 Regioselectivity''': Probability to be metabolized in human liver microsomes (or by a specific | # '''P450 Regioselectivity''': Probability to be metabolized in human liver microsomes (or by a specific cytochrome P450 isoform) for every atom in the molecule. | ||
Predictions are provided for five major cytochrome P450 isoforms (3A4, 2D6, 2C9, 2C19, 1A2) that are responsible for more than 80% of Phase I metabolism. In addition to Regioselectivity models for individual enzymes, overall HLM Regioselectivity module is also available. This module estimates the overall probabilities of human liver microsomal metabolism taking place at particular sites of the molecule. All predictions are supplied with Reliability Indices (RI) serving as an internal measure of prediction confidence (see Model Features section for more details about RI calculation). | Predictions are provided for five major cytochrome P450 isoforms (3A4, 2D6, 2C9, 2C19, 1A2) that are responsible for more than 80% of Phase I metabolism. In addition to Regioselectivity models for individual enzymes, overall HLM Regioselectivity module is also available. This module estimates the overall probabilities of human liver microsomal metabolism taking place at particular sites of the molecule. All predictions are supplied with Reliability Indices (''RI'') serving as an internal measure of prediction confidence (see Model Features section for more details about ''RI'' calculation). | ||
===Sources of experimental data=== | ===Sources of experimental data=== | ||
# '''P450 Metabolism''' (Substrate specificity and metabolism sites): only experimental data from original scientific publications were used for modeling of cytochrome P450 | # '''P450 Metabolism''' (Substrate specificity and metabolism sites): only experimental data from original scientific publications were used for modeling of cytochrome P450 metabolism sites. The literature dataset was expanded with information about marketed drugs’ metabolism and the expanded dataset was used for cytochrome P450 substrate modeling. Two main types of assays were considered: | ||
# '''P450 Inhibition''': | #* Metabolism experiments using recombinant human cytochrome P450 enzymes | ||
#* Analysis of human liver microsomal (HLM) metabolism with contributions of a particular P450 isoform evaluated by evidence of metabolism inhibition with specific inhibitors of that isoform. | |||
# '''P450 Inhibition''': Inhibitor specificity data were also collected from original scientific publications based on HLM/recombinant enzyme assays, focusing on inhibition of metabolism of standard probe substrates by test compounds. Additionally, these databases were supplemented with information about marketed drugs, as well as HTS assay data from NCBI PubChem project. | |||
===Data sets=== | ===Data sets=== | ||
| Line 94: | Line 96: | ||
|} | |} | ||
Fully searchable | Fully searchable Cytochrome P450 Specificity databases are not available in the current version of ACD/Percepta, yet each prediction performed by P450 Substrates and Inhibitors modules is displayed along with experimental data for five compounds from the training set most similar to the molecule of interest. The provided information for similar compounds includes classification as substrates/non-substrates (inhibitors/non-inhibitors at two IC50 cut-offs) of the relevant cytochrome P450 enzyme assigned on the basis of experimental results together with original references. In case of Regioselectivity predictions five most similar atoms from the training set are shown with color-marks indicating whether a metabolic reaction taking place at the particular site of the molecules was observed experimentally. | ||
===Model features & prediction accuracy=== | ===Model features & prediction accuracy=== | ||
The | The predictive models of all cytochrome P450-related endpoints were derived using GALAS (Global, Adjusted Locally According to Similarity) modeling methodology (please refer to [https://www.ncbi.nlm.nih.gov/pubmed/20373217] for more details). | ||
Each GALAS model consists of two parts: | |||
* Global baseline statistical model employing binomial PLS with multiple bootstrapping using a predefined set of fragmental descriptors, that reflects general trends in the considered property. | |||
* Similarity-based routine that performs local correction of baseline predictions taking into account the differences between baseline and experimental values for the most similar training set compounds. | |||
<br> | |||
GALAS methodology also provides the basis for estimating reliability of predictions by the means of calculated Reliability Index (''RI'') value that takes into account: | |||
* Similarity of tested compound to the training set molecules (prediction is unreliable if no similar compounds have been found). | |||
* Consistence of experimental values and baseline model prediction for the most similar similar compounds from the training set (discrepant data for similar molecules lead to lower ''RI'' values). | |||
If the compound is within model Applicability Domain (acceptable | Reliability Index ranges from 0 to 1 (0 corresponds to a completely unreliable, and 1 - a highly reliable prediction) and serves as an indication whether a submitted compound falls within the Model Applicability Domain. Compounds obtaining predictions ''RI'' < 0.3 are considered outside of the Applicability Domain of the model. | ||
<br><br> | |||
The predictive models of cytochrome P450 substrate and inhibitor specificity are also '''Trainable''' meaning that their Applicability Domains may be expanded to account for the ‘in-house’ experimental data available in your company without the need to rebuild the baseline statistical model from scratch. Addition of new compounds to the module Self-training Library results in an instant improvement of prediction accuracy for the respective compound classes. Moreover, addition of 'in-house' data allows adapting the existing model to the particular experimental protocol used in your company and avoiding potential issues related to discrepancies between different experimental methods used for determination of drug interactions with CYP450 enzymes. | |||
If the compound is within model Applicability Domain (acceptable Reliability Index) accuracy and sensitivity of classification is close to 90% for inhibitors and close to 80% for substrates. The accuracy of ''in silico'' prediction of cytochrome P450 inhibition is comparable to the screening results. | |||
The results of the external validation of CYP3A4 Inhibition prediction model and a demonstration of model training can be found in an Application Note [http://perceptahelp.acdlabs.com/docs/1007_CYP3A4_validation.pdf]. | |||
For information on the competitive performance of HLM Regioselectivity model please refer to [http://perceptahelp.acdlabs.com/docs/1006_p450regioselectivity.pdf]. | |||
===Publications=== | |||
More detailed information about the construction of databases, and application of GALAS method to modeling cytochrome P450-related properties can be found in the following articles: | |||
* Regioselectivity of metabolism: Dapkunas J. et al. ''Chem Biodivers.'' '''2009''';6(11):2101-6 [https://www.ncbi.nlm.nih.gov/pubmed/19937844] | |||
* Inhibition of metabolism: Didziapetris R. et al. ''J Comput Aided Mol Des.'' '''2010''';24(11):891-906 [https://www.ncbi.nlm.nih.gov/pubmed/20814717] | |||
Latest revision as of 08:18, 20 October 2022
Overview
The information about possible metabolites of a drug candidate is desirable in the earliest stages of drug development, but the experimental testing of biotransformations is usually done only in the later phases. The P450 regioselectivity module of ACD/Percepta predicts possible sites of metabolic reactions taking place in human liver microsomes (HLM) and catalyzed by 5 individual cytochrome P450 enzymes (CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4). The predictive models are based on experimental data for >900 compounds. Metabolites that are formed when incubating the compound with human liver microsomes and recombinant enzymes were considered. Separate probabilistic models were built for 5 different reaction types: aliphatic and aromatic hydroxylation, N-dealkylation, O-dealkylation and S-oxidation.
Six separate regioselectivity modules are provided. "Human Liver Microsomes" module identifies all probable sites of human liver microsomal metabolism in the molecule while the other modules predict regioselectivity of the reactions catalyzed by individual enzymes (CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4).
Features
- Predicts possibility of being a metabolism site for every atom in the molecule
- Calculates a Reliability Index for every prediction
- Illustrates the predictions in a color-coded manner
- Performs a similarity search and displays top 5 most similar structures from the training set of the model along with their names and experimental results
Interface
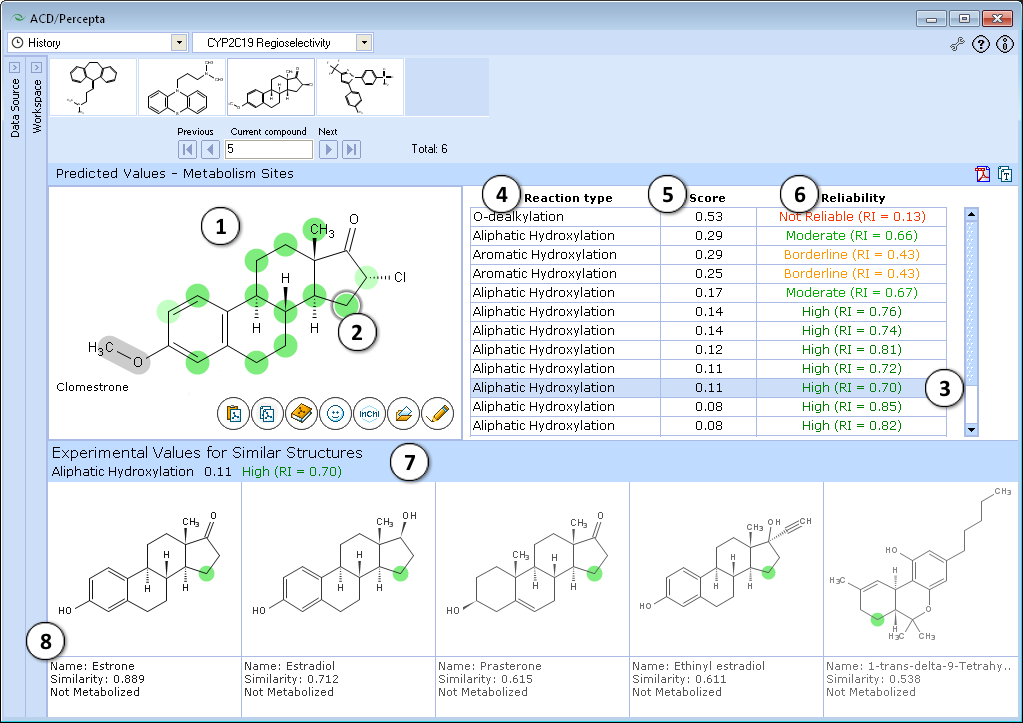
- Predictions are performed for all possible metabolism sites in the molecule and atoms are colored according to predicted values: intensive red corresponds to probability > 0.8; light red – probability in the range 0.6-0.8. Green color indicates non-metabolized atoms (intensive green – probability < 0.2, light green – probability in the range 0.2-0.4), while inconclusive predictions (probability in the range 0.4-0.6) are marked gray.
- Atom with the highest predicted score of metabolism is automatically selected and marked with a circle. Other atoms can be selected by clicking on them or by clicking on the corresponding entry in the table in the right
- Table entry corresponding to currently selected atom is highlighted
- Type of metabolic reaction taking place at the particular site
- Calculated score for an atom to be a metabolism site
- Calculated value of Reliability Index (RI) supporting the prediction
- Prediction for the currently selected atom is outlined on the top of Similar Structures Pane
- Five most similar atoms from the training set are displayed along with experimentally assigned classes and Similarity Indices to the selected atom. Similar atoms are color-marked according to experimental data (red – experimentally determined site of metabolism, green – no metabolism observed, grey - inconclusive data).
Technical information
Predicted endpoints
Cytochrome P450 Specificity-related modules in ACD/Percepta provide the following quantitative predictions:
- P450 Substrates: Probability that the compound of interest will be metabolized by a certain cytochrome P450 isoform.
- P450 Inhibitors: Probability that the compound of interest will inhibit a certain cytochrome P450 enzyme with IC50 below defined threshold. Two types of predictive models utilizing different IC50 thresholds have been developed. "General inhibition" models estimate whether the analyzed compound will exhibit any clinically significant cytochrome P450 inhibition at all (IC50 < 50 μM), while "Efficient inhibition" models predict probability that the compound will inhibit selected enzyme with IC50 < 10 μM.
- P450 Regioselectivity: Probability to be metabolized in human liver microsomes (or by a specific cytochrome P450 isoform) for every atom in the molecule.
Predictions are provided for five major cytochrome P450 isoforms (3A4, 2D6, 2C9, 2C19, 1A2) that are responsible for more than 80% of Phase I metabolism. In addition to Regioselectivity models for individual enzymes, overall HLM Regioselectivity module is also available. This module estimates the overall probabilities of human liver microsomal metabolism taking place at particular sites of the molecule. All predictions are supplied with Reliability Indices (RI) serving as an internal measure of prediction confidence (see Model Features section for more details about RI calculation).
Sources of experimental data
- P450 Metabolism (Substrate specificity and metabolism sites): only experimental data from original scientific publications were used for modeling of cytochrome P450 metabolism sites. The literature dataset was expanded with information about marketed drugs’ metabolism and the expanded dataset was used for cytochrome P450 substrate modeling. Two main types of assays were considered:
- Metabolism experiments using recombinant human cytochrome P450 enzymes
- Analysis of human liver microsomal (HLM) metabolism with contributions of a particular P450 isoform evaluated by evidence of metabolism inhibition with specific inhibitors of that isoform.
- P450 Inhibition: Inhibitor specificity data were also collected from original scientific publications based on HLM/recombinant enzyme assays, focusing on inhibition of metabolism of standard probe substrates by test compounds. Additionally, these databases were supplemented with information about marketed drugs, as well as HTS assay data from NCBI PubChem project.
Data sets
The sizes of the data sets used to develop the predictive models of substrate and inhibitor specificity are presented in the table below:
| Isoform | N (Substrate specificity) | N (Inhibitor specificity, cut-off: IC50 < 50 μM) | N (Efficient inhibition, cut-off: IC50 < 10 μM) |
|---|---|---|---|
| CYP1A2 | 935 | 4867 | 5815 |
| CYP2C9 | 867 | 7666 | 7677 |
| CYP2C19 | 794 | 6899 | 6833 |
| CYP2D6 | 1001 | 7707 | 7507 |
| CYP3A4 | 960 | 6684 | 7927 |
Regioselectivity models were based on experimental data for 873 compounds collected from publications dealing with analytical identification of the metabolites observed after the incubation of compound with human liver microsomes or recombinant cytochrome P450 enzymes. Every carbon atom with at least one hydrogen attached was marked as a site of metabolism, if hydroxylation at the atom was observed, or site of no metabolism otherwise. For dealkylation reactions, carbon atoms of the leaving groups were marked in the same manner. Some sites were marked as "inconclusive" and consequently not used in the modeling. The table below shows the overall number of marked atoms used for building the models:
| No. of atoms | HLM | CYP3A4 | CYP2D6 | CYP2C9 | CYP2C19 | CYP1A2 |
|---|---|---|---|---|---|---|
| Positive | 1269 | 795 | 354 | 288 | 249 | 383 |
| Inconclusive | 340 | 176 | 49 | 43 | 15 | 61 |
| Negative | 7182 | 6757 | 6305 | 6314 | 5210 | 6020 |
| Total | 8791 | 7728 | 6708 | 6645 | 5474 | 6464 |
Fully searchable Cytochrome P450 Specificity databases are not available in the current version of ACD/Percepta, yet each prediction performed by P450 Substrates and Inhibitors modules is displayed along with experimental data for five compounds from the training set most similar to the molecule of interest. The provided information for similar compounds includes classification as substrates/non-substrates (inhibitors/non-inhibitors at two IC50 cut-offs) of the relevant cytochrome P450 enzyme assigned on the basis of experimental results together with original references. In case of Regioselectivity predictions five most similar atoms from the training set are shown with color-marks indicating whether a metabolic reaction taking place at the particular site of the molecules was observed experimentally.
Model features & prediction accuracy
The predictive models of all cytochrome P450-related endpoints were derived using GALAS (Global, Adjusted Locally According to Similarity) modeling methodology (please refer to [1] for more details).
Each GALAS model consists of two parts:
- Global baseline statistical model employing binomial PLS with multiple bootstrapping using a predefined set of fragmental descriptors, that reflects general trends in the considered property.
- Similarity-based routine that performs local correction of baseline predictions taking into account the differences between baseline and experimental values for the most similar training set compounds.
GALAS methodology also provides the basis for estimating reliability of predictions by the means of calculated Reliability Index (RI) value that takes into account:
- Similarity of tested compound to the training set molecules (prediction is unreliable if no similar compounds have been found).
- Consistence of experimental values and baseline model prediction for the most similar similar compounds from the training set (discrepant data for similar molecules lead to lower RI values).
Reliability Index ranges from 0 to 1 (0 corresponds to a completely unreliable, and 1 - a highly reliable prediction) and serves as an indication whether a submitted compound falls within the Model Applicability Domain. Compounds obtaining predictions RI < 0.3 are considered outside of the Applicability Domain of the model.
The predictive models of cytochrome P450 substrate and inhibitor specificity are also Trainable meaning that their Applicability Domains may be expanded to account for the ‘in-house’ experimental data available in your company without the need to rebuild the baseline statistical model from scratch. Addition of new compounds to the module Self-training Library results in an instant improvement of prediction accuracy for the respective compound classes. Moreover, addition of 'in-house' data allows adapting the existing model to the particular experimental protocol used in your company and avoiding potential issues related to discrepancies between different experimental methods used for determination of drug interactions with CYP450 enzymes.
If the compound is within model Applicability Domain (acceptable Reliability Index) accuracy and sensitivity of classification is close to 90% for inhibitors and close to 80% for substrates. The accuracy of in silico prediction of cytochrome P450 inhibition is comparable to the screening results.
The results of the external validation of CYP3A4 Inhibition prediction model and a demonstration of model training can be found in an Application Note [2]. For information on the competitive performance of HLM Regioselectivity model please refer to [3].
Publications
More detailed information about the construction of databases, and application of GALAS method to modeling cytochrome P450-related properties can be found in the following articles: