Absorption: Difference between revisions
No edit summary |
|||
| (14 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
==Overview== | ==Overview== | ||
Human intestinal | Human intestinal Absorption (HIA) of drugs together with solubility are the two key factors affecting their oral bioavailability. HIA may be defined as a drug passing from the lumen into the tissue of the gastrointestinal tract [http://www.ncbi.nlm.nih.gov/pubmed/2654032?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum]. Once in the tissue, the drug is considered absorbed. Absorption predictor in ACD/Percepta analyzes HIA in terms of passive permeability that is not affected by any side processes such as limited solubility/dissolution, variable oral dose, chemical stability, active transport, and 1st pass metabolism in gut or liver.<br> | ||
Absorption module provides accurate predictions of passive intestinal permeability (on jejunal and Caco-2 scales) and extent of oral absorption (%HIA) of drug candidates. These predictions based on intuitive, easily interpretable physicochemical models enable the researchers to rank and select lead compounds according to their permeability across intestinal barrier and to exclude candidates exhibiting extremely poor absorption at the earliest stages. | |||
===Features=== | ===Features=== | ||
* Calculates the extent of oral absorption of analyzed compounds as well as their passive permeability across jejunal epithelium using values of their physicochemical properties such as lipophilicity (LogP) and ionization (pKa) as inputs. | * Calculates the extent of oral absorption of analyzed compounds as well as their passive permeability across jejunal epithelium using values of their physicochemical properties such as lipophilicity (LogP) and ionization (pKa) as inputs. | ||
* Gives an estimate of relative contributions of different transport routes | * Gives an estimate of relative contributions of different transport routes to overall %HIA. | ||
* Allows entering experimentally measured physicochemical properties instead of automatically calculated values to improve the quality of predictions. | * Allows entering experimentally measured physicochemical properties instead of automatically calculated values to improve the quality of predictions. | ||
* Entering user-defined LogP and pKa values allows the researcher to model the limiting effect of lipophilicity and ionization on intestinal permeation rate, thus providing a straightforward route for property-based design of oral drugs. | * Entering user-defined LogP and pKa values allows the researcher to model the limiting effect of lipophilicity and ionization on intestinal permeation rate, thus providing a straightforward route for property-based design of oral drugs. | ||
* Provides access to Absorption DB – a fully browsable and searchable database containing experimental data that was used for the development of HIA model together with corresponding literature references. | * Provides access to Absorption DB – a fully browsable and searchable database containing experimental data that was used for the development of HIA model together with corresponding literature references. | ||
* Displays the experimental values of the relevant properties for up to 3 similar structures from Absorption DB along with each HIA prediction. | * Displays the experimental values of the relevant properties for up to 3 similar structures from Absorption DB along with each HIA prediction.<br /> | ||
<br /> | |||
== Interface == | == Interface == | ||
<br /> | |||
[[Image:Absorption_HIA.png|center]] | [[Image:Absorption_HIA.png|center]] | ||
<br /> | |||
<ol> | <ol> | ||
| Line 25: | Line 28: | ||
:b. Click to recalculate the maximum passive absorption, permeability and absorption rate values using the currently specified logP and pKa values | :b. Click to recalculate the maximum passive absorption, permeability and absorption rate values using the currently specified logP and pKa values | ||
</li> | </li> | ||
<li>Quantitative estimate of maximum intestinal passive absorption of a compound and the relative contributions from the transcellular and paracellular routes of absorption, calculated as a function of compound structure, lipophilicity and ionization constants</li> | <li>Quantitative estimate of maximum intestinal passive absorption of a compound and the relative contributions from the transcellular and paracellular routes of absorption, calculated as a function of compound structure, lipophilicity and ionization constants<br>[[File:Absorption_hia_results.png|400px]]</li> | ||
<li>Estimated human | <li>Estimated human jejunal permeability (Pe), in cm/s, and calculated intestinal absorption rate constant (k<sub>a</sub>) in units of 1/min</li> | ||
<li>Up to 3 most similar structures in the '''Absorption DB''' with experimental values and references</li> | <li>Up to 3 most similar structures in the '''Absorption DB''' with experimental values and references</li> | ||
</ol> | </ol> | ||
<br /> | |||
<div class="mw-collapsible | <div class="mw-collapsible"> | ||
==Technical information== | ==Technical information== | ||
<br /> | <br /> | ||
<div class="mw-collapsible-content"> | <div class="mw-collapsible-content"> | ||
Only a short summary of main technical aspects of Absorption predictor is given here. For a more detailed description of the modeling approach and underlying theory please refer to | Only a short summary of main technical aspects of Human Intestinal Absorption predictor is given here. For a more detailed description of the modeling approach and underlying theory please refer to ''J Pharm Sci.'' '''2009''';98(11):4039-54. [https://doi.org/10.1002/jps.21730] | ||
Caco-2 permeability predictor was developed using the same general principles. However, this model has been recently re-parameterized using a larger data set and a simplified approach based on a composite LogD descriptor instead of separate LogP and pKa parameters. More information about the new Caco-2 permeability model can be found in ''J Pharm Sci.'' '''2019''';108(1):78-86. [https://doi.org/10.1016/j.xphs.2018.10.006] | |||
===Calculated quantitative parameters=== | ===Calculated quantitative parameters=== | ||
The main output of the Absorption module is the maximum achievable extent of human intestinal absorption (when solubility is not a limiting factor) expressed as a percentage value and denoted %HIA. Typically, compounds exhibiting %HIA > 70% are considered well absorbed, those with %HIA < 30% - poorly absorbed, while values in the range 30 - 70% represent moderate absorption. Intestinal permeability rates corresponding to the respective %HIA values are calibrated on several scales and given as: | <br /> | ||
The main output of the Passive Absorption module is the maximum achievable extent of human intestinal absorption (when solubility is not a limiting factor) expressed as a percentage value and denoted %HIA. Typically, compounds exhibiting %HIA > 70% are considered well absorbed, those with %HIA < 30% - poorly absorbed, while values in the range 30 - 70% represent moderate absorption. Intestinal permeability rates corresponding to the respective %HIA values are calibrated on several scales and given as: | |||
* Effective jejunal permeability coefficients at pH 6.5 (''P<sub>e</sub>'', 10<sup>-4</sup> cm/s) | * Effective jejunal permeability coefficients at pH 6.5 (''P<sub>e</sub>'', 10<sup>-4</sup> cm/s) | ||
* Absorption rate constants (''k<sub>a</sub>'', min<sup>-1</sup>). | * Absorption rate constants (''k<sub>a</sub>'', min<sup>-1</sup>). | ||
Additionally, Absorption\Caco-2 module presents effective permeability coefficients in Caco-2 monolayers (''P<sub>e</sub>'', 10<sup>-6</sup> cm/s) at user-defined conditions (pH and stirring rate). | Additionally, Passive Absorption\Caco-2 module presents effective permeability coefficients in Caco-2 monolayers (''P<sub>e</sub>'', 10<sup>-6</sup> cm/s) at user-defined conditions (pH and stirring rate). | ||
===Descriptors & Modeling Method=== | ===Descriptors & Modeling Method=== | ||
Descriptors used for modeling included key physicochemical properties - octanol/water LogP of neutral species as a determinant of lipophilicity, ion form fractions at pH 6.5 calculated from the respective pKa values, number of hydrogen bond donors in the molecule, and McGowan | <br /> | ||
Descriptors used for modeling included key physicochemical properties - octanol/water LogP of neutral species as a determinant of lipophilicity, ion form fractions at pH 6.5 calculated from the respective pKa values, number of hydrogen bond donors in the molecule, and McGowan characteristic volume reflecting molecular size. All physicochemical parameter values were calculated with Algorithm Builder software (''Quant Struct Act Relat.'' '''2002''';21(1):23-37. [https://doi.org/10.1002/1521-3838(200205)21:1%3C23::AID-QSAR23%3E3.0.CO;2-E]) | |||
Due to the evidence of sigmoidal relationship between fraction absorbed (represented by experimental data) and actual permeation rates data were modeled using non-linear least squares regression. Fitting was performed in a multi-step approach | Due to the evidence of sigmoidal relationship between fraction absorbed (represented by experimental data) and actual permeation rates data were modeled using non-linear least squares regression. Fitting was performed in a multi-step approach with separate stages employed for: | ||
* Determination of paracellular transport parameters | * Determination of paracellular transport parameters | ||
| Line 60: | Line 68: | ||
* Estimating ionization dependence of intestinal absorption | * Estimating ionization dependence of intestinal absorption | ||
The sigmoid relationships between %HIA and octanol/water log ''P'' for various electrolyte classes are illustrated in the figure below. The sigmoids obtained for charged species are shifted relatively to the respective curve for non-electrolytes, although the shift is not as marked as could be expected if absorption was modeled by pH-dependent octanol/water distribution coefficient log ''D'' | The sigmoid relationships between %HIA and octanol/water log ''P'' for various electrolyte classes are illustrated in the figure below. The sigmoids obtained for charged species are shifted relatively to the respective curve for non-electrolytes, although the shift is not as marked as could be expected if absorption was modeled by pH-dependent octanol/water distribution coefficient log ''D''. Ionization-specific analysis was therefore performed to devise more precise corrections to log ''P'' values needed for prediction of passive jejunal permeability. | ||
[[Image:HIA_chart.gif|frame|none|Non-linear dependence of fraction absorbed on lipophilicity for compounds representing different ionization states. Only compounds with MW > 250 were considered to minimize the contribution from paracellular absorption route). Bases: pK<sub>a</sub><sup>B</sup> > 7, acids: pK<sub>a</sub><sup>A</sup> < 5.]] | [[Image:HIA_chart.gif|frame|none|Non-linear dependence of fraction absorbed on lipophilicity for compounds representing different ionization states. Only compounds with MW > 250 were considered to minimize the contribution from paracellular absorption route). Bases: pK<sub>a</sub><sup>B</sup> > 7, acids: pK<sub>a</sub><sup>A</sup> < 5.]] | ||
In case of Caco-2 permeability, analysis of the new expanded data set including many representatives of novel pharmaceutical classes revealed even less pronounced differences to octanol/water system in terms of the effect of ionization. Accordingly, no significant differences in prediction accuracy were observed between models based on separate log ''P''/ion form fraction descriptors, or a single log ''D'' descriptor accounting for both lipophilicity and ionization influence. Therefore, the latter approach was preferred due to simplicity, and considering the fact that in many recent publications lipophilicity of novel chemicals is determined as log ''D'' at relevant pH. Nevertheless, the current implementation of Caco-2 permeability predictor in ACD/Percepta allows for a more flexible representation of physicochemical properties and accepts either log ''D'', or log ''P'' + pKa values as inputs for calculation. In the latter case log ''P'' is recalculated to log ''D'', which is then supplied to Caco-2 model. | |||
===Prediction accuracy=== | ===Prediction accuracy=== | ||
The final quantitative %HIA data set (cleaned of values distorted by P-gp efflux, facilitated diffusion and other side processes) that was used for modeling consisted of 567 %HIA values, mostly for marketed drugs or drug candidates. For validation purposes two independent test sets were compiled from | The final quantitative %HIA data set (cleaned of values distorted by P-gp efflux, facilitated diffusion and other side processes) that was used for modeling consisted of 567 %HIA values, mostly for marketed drugs or drug candidates. For validation purposes two independent test sets were compiled from several publications dealing with jejunal absorption of drugs. External validation sets represented other types of experimental data that were not used in model development: | ||
* The first set contained directly measured jejunal permeability coefficients (''P<sub>eff</sub>'') for 25 compounds extracted from | * The first set contained directly measured jejunal permeability coefficients (''P<sub>eff</sub>'') for 25 compounds extracted from ''Xenobiotica.'' '''2007''';37(10-11):1015-51. [https://doi.org/10.1080/00498250701704819] | ||
* The second set was comprised of absorption rate constants (''k<sub>a</sub>'') for 22 molecules from | * The second set was comprised of absorption rate constants (''k<sub>a</sub>'') for 22 molecules from ''J Med Chem.'' '''2006''';49(12):3674-81. [https://doi.org/10.1021/jm051231p]. | ||
The main advantage of such type of validation is the possibility to evaluate the intrinsic correctness of our model rather than just goodness of fit between experimental and predicted HIA. | The main advantage of such type of validation is the possibility to evaluate the intrinsic correctness of our model rather than just goodness of fit between experimental and predicted HIA. | ||
| Line 90: | Line 99: | ||
|} | |} | ||
Note that RMSE for the training set is grayed since prediction error for percentage values (that are mostly concentrated on the ends of the scale) does not provide an | Note that RMSE for the training set is grayed since prediction error for percentage values (that are mostly concentrated on the ends of the scale) does not provide an unambiguous measure of model quality, while the actual accuracy of predictions is best illustrated by model performance on external validation sets yielding RMSE about 0.4 log units which is close to the error of experimental determination.<br /> | ||
The respective results for Caco-2 permeability model are as follows: | |||
{| class="wikitable" | |||
|- | |||
! style="border-top:2px; border-bottom:2px; background:silver;" width="200" | Data set | |||
! style="border-top:2px; border-bottom:2px; background:silver;" width="100" | N | |||
! style="border-top:2px; border-bottom:2px; background:silver;" width="100" | R<sup>2</sup> | |||
! style="border-top:2px; border-bottom:2px; background:silver;" width="100" | RMSE | |||
|- | |||
| Training set || style="text-align:right;" | 497 || style="text-align:right;" | 0.80 || style="text-align:right;" | 0.44 | |||
|- | |||
| Internal validation set || style="text-align:right;" | 442 || style="text-align:right;" | 0.77 || style="text-align:right;" | 0.49 | |||
|- | |||
| External validation set || style="text-align:right;" | 427 || style="text-align:right;" | 0.53 || style="text-align:right;" | 0.47 | |||
|} | |||
A lower R<sup>2</sup> value was obtained for external validation set because this set had a much narrower variation | |||
range of both log ''D'', and log ''Pe'' values. Nevertheless, consistently low RMSE values indicate that the model is not-overfitted and can predict passive permeability close to the level of experimental uncertainty. | |||
===Reference database=== | ===Reference database=== | ||
In addition to HIA and Caco-2 permeability predictions, ACD/Percepta provides Absorption DB - a a browsable database of human intestinal absorption & bioavailability. The experimental data were compiled from reference pharmacokinetic tabulations and original articles, the main sources being "Therapeutic Drugs" (ed. by C. Dollery), Goodman & Gilman's "The Pharmacological Basis of Therapeutics", and a compilation by Zhao, Y. H. et al. ''J Pharm Sci.'' '''2001''';90(6):749-84. [https://doi.org/10.1002/jps.1031] A qualitative assignment of absorption category (good, moderate, poor) is provided for each compound in the database along with comments regarding the quantitative extent of absorption/bioavailability, presence of carrier-mediated transport, etc. where available. | |||
<br /> | |||
===Transport mechanisms=== | ===Transport mechanisms=== | ||
The predictive models comprising the Absorption module account for '''passive''' transport of analyzed compounds across intestinal barrier. In order to propose a clear physicochemical explanation of passive diffusion process, the data used for model development | The predictive models comprising the Passive Absorption module account for '''passive''' transport of analyzed compounds across intestinal barrier. In order to propose a clear physicochemical explanation of passive diffusion process, the data used for model development were thoroughly evaluated to exclude values affected by enzymatic efflux or influx. For such compounds special alerts are displayed in the Oral Bioavailability module indicating that expected oral bioavailability/absorption values for these molecules may be higher or lower due to presence of carrier-mediated processes. | ||
</div> | </div> | ||
</div> | </div> | ||
Latest revision as of 08:07, 20 August 2020
Overview
Human intestinal Absorption (HIA) of drugs together with solubility are the two key factors affecting their oral bioavailability. HIA may be defined as a drug passing from the lumen into the tissue of the gastrointestinal tract [1]. Once in the tissue, the drug is considered absorbed. Absorption predictor in ACD/Percepta analyzes HIA in terms of passive permeability that is not affected by any side processes such as limited solubility/dissolution, variable oral dose, chemical stability, active transport, and 1st pass metabolism in gut or liver.
Absorption module provides accurate predictions of passive intestinal permeability (on jejunal and Caco-2 scales) and extent of oral absorption (%HIA) of drug candidates. These predictions based on intuitive, easily interpretable physicochemical models enable the researchers to rank and select lead compounds according to their permeability across intestinal barrier and to exclude candidates exhibiting extremely poor absorption at the earliest stages.
Features
- Calculates the extent of oral absorption of analyzed compounds as well as their passive permeability across jejunal epithelium using values of their physicochemical properties such as lipophilicity (LogP) and ionization (pKa) as inputs.
- Gives an estimate of relative contributions of different transport routes to overall %HIA.
- Allows entering experimentally measured physicochemical properties instead of automatically calculated values to improve the quality of predictions.
- Entering user-defined LogP and pKa values allows the researcher to model the limiting effect of lipophilicity and ionization on intestinal permeation rate, thus providing a straightforward route for property-based design of oral drugs.
- Provides access to Absorption DB – a fully browsable and searchable database containing experimental data that was used for the development of HIA model together with corresponding literature references.
- Displays the experimental values of the relevant properties for up to 3 similar structures from Absorption DB along with each HIA prediction.
Interface
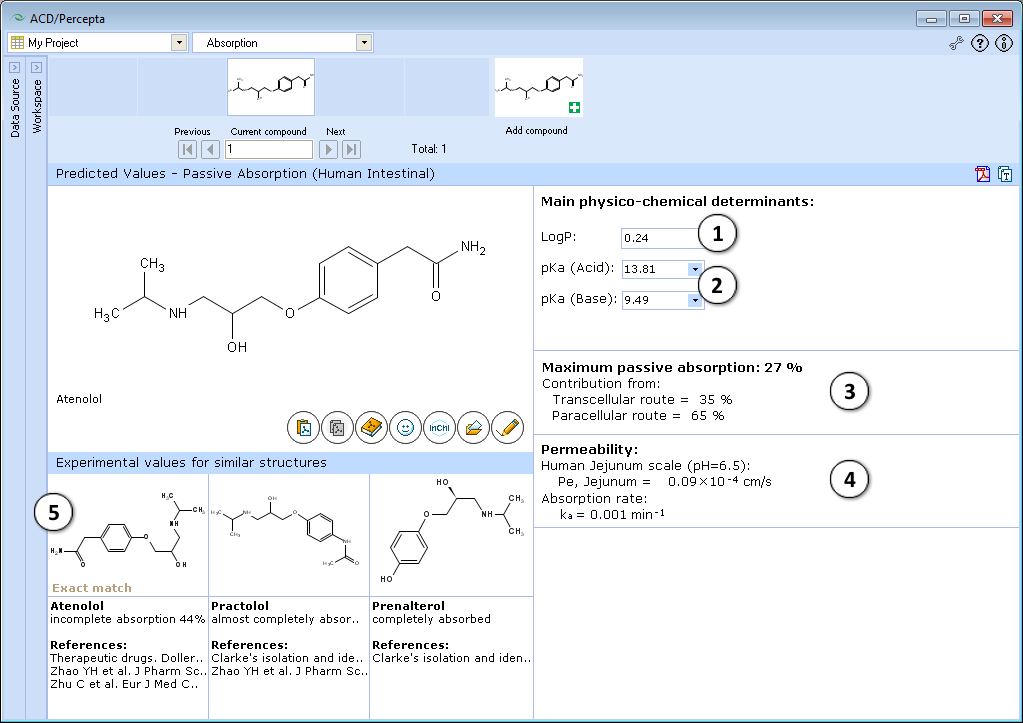
- Calculated logP. Click and type a new value to model the limiting effect of lipophilicity on maximum absorption and intestinal permeability
- Calculated acid and base ionization constants. Click to select or type new pKa(acid) and pKa(base) values to model the limiting effect of ionization constants on maximum absorption and intestinal permeability
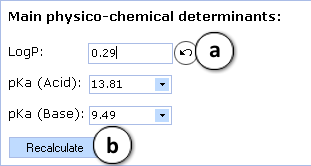
- a. Click to reverse to an automatically calculated property value (logP in this picture) for a compound and to recalculate the maximum passive absorption, permeability and absorption rate values
- b. Click to recalculate the maximum passive absorption, permeability and absorption rate values using the currently specified logP and pKa values
- Quantitative estimate of maximum intestinal passive absorption of a compound and the relative contributions from the transcellular and paracellular routes of absorption, calculated as a function of compound structure, lipophilicity and ionization constants
- Estimated human jejunal permeability (Pe), in cm/s, and calculated intestinal absorption rate constant (ka) in units of 1/min
- Up to 3 most similar structures in the Absorption DB with experimental values and references
Technical information
Only a short summary of main technical aspects of Human Intestinal Absorption predictor is given here. For a more detailed description of the modeling approach and underlying theory please refer to J Pharm Sci. 2009;98(11):4039-54. [2]
Caco-2 permeability predictor was developed using the same general principles. However, this model has been recently re-parameterized using a larger data set and a simplified approach based on a composite LogD descriptor instead of separate LogP and pKa parameters. More information about the new Caco-2 permeability model can be found in J Pharm Sci. 2019;108(1):78-86. [3]
Calculated quantitative parameters
The main output of the Passive Absorption module is the maximum achievable extent of human intestinal absorption (when solubility is not a limiting factor) expressed as a percentage value and denoted %HIA. Typically, compounds exhibiting %HIA > 70% are considered well absorbed, those with %HIA < 30% - poorly absorbed, while values in the range 30 - 70% represent moderate absorption. Intestinal permeability rates corresponding to the respective %HIA values are calibrated on several scales and given as:
- Effective jejunal permeability coefficients at pH 6.5 (Pe, 10-4 cm/s)
- Absorption rate constants (ka, min-1).
Additionally, Passive Absorption\Caco-2 module presents effective permeability coefficients in Caco-2 monolayers (Pe, 10-6 cm/s) at user-defined conditions (pH and stirring rate).
Descriptors & Modeling Method
Descriptors used for modeling included key physicochemical properties - octanol/water LogP of neutral species as a determinant of lipophilicity, ion form fractions at pH 6.5 calculated from the respective pKa values, number of hydrogen bond donors in the molecule, and McGowan characteristic volume reflecting molecular size. All physicochemical parameter values were calculated with Algorithm Builder software (Quant Struct Act Relat. 2002;21(1):23-37. [4])
Due to the evidence of sigmoidal relationship between fraction absorbed (represented by experimental data) and actual permeation rates data were modeled using non-linear least squares regression. Fitting was performed in a multi-step approach with separate stages employed for:
- Determination of paracellular transport parameters
- Describing trancellular diffusion of non-electrolytes
- Estimating ionization dependence of intestinal absorption
The sigmoid relationships between %HIA and octanol/water log P for various electrolyte classes are illustrated in the figure below. The sigmoids obtained for charged species are shifted relatively to the respective curve for non-electrolytes, although the shift is not as marked as could be expected if absorption was modeled by pH-dependent octanol/water distribution coefficient log D. Ionization-specific analysis was therefore performed to devise more precise corrections to log P values needed for prediction of passive jejunal permeability.
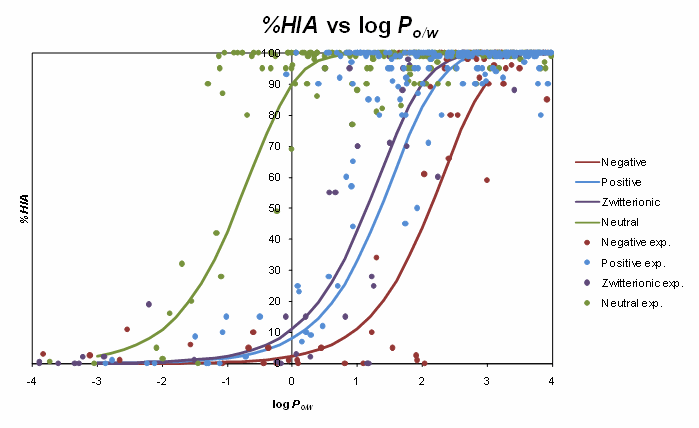
In case of Caco-2 permeability, analysis of the new expanded data set including many representatives of novel pharmaceutical classes revealed even less pronounced differences to octanol/water system in terms of the effect of ionization. Accordingly, no significant differences in prediction accuracy were observed between models based on separate log P/ion form fraction descriptors, or a single log D descriptor accounting for both lipophilicity and ionization influence. Therefore, the latter approach was preferred due to simplicity, and considering the fact that in many recent publications lipophilicity of novel chemicals is determined as log D at relevant pH. Nevertheless, the current implementation of Caco-2 permeability predictor in ACD/Percepta allows for a more flexible representation of physicochemical properties and accepts either log D, or log P + pKa values as inputs for calculation. In the latter case log P is recalculated to log D, which is then supplied to Caco-2 model.
Prediction accuracy
The final quantitative %HIA data set (cleaned of values distorted by P-gp efflux, facilitated diffusion and other side processes) that was used for modeling consisted of 567 %HIA values, mostly for marketed drugs or drug candidates. For validation purposes two independent test sets were compiled from several publications dealing with jejunal absorption of drugs. External validation sets represented other types of experimental data that were not used in model development:
- The first set contained directly measured jejunal permeability coefficients (Peff) for 25 compounds extracted from Xenobiotica. 2007;37(10-11):1015-51. [5]
- The second set was comprised of absorption rate constants (ka) for 22 molecules from J Med Chem. 2006;49(12):3674-81. [6].
The main advantage of such type of validation is the possibility to evaluate the intrinsic correctness of our model rather than just goodness of fit between experimental and predicted HIA.
Model performance on internal %HIA data set and external validation sets is summarized in the table below:
| Data set | N | R2 | RMSE |
|---|---|---|---|
| %HIA training set | 567 | 0.93 | 9.5% |
| log Peff validation set | 25 | 0.72 | 0.45 |
| log Ka validation set | 22 | 0.84 | 0.35 |
Note that RMSE for the training set is grayed since prediction error for percentage values (that are mostly concentrated on the ends of the scale) does not provide an unambiguous measure of model quality, while the actual accuracy of predictions is best illustrated by model performance on external validation sets yielding RMSE about 0.4 log units which is close to the error of experimental determination.
The respective results for Caco-2 permeability model are as follows:
| Data set | N | R2 | RMSE |
|---|---|---|---|
| Training set | 497 | 0.80 | 0.44 |
| Internal validation set | 442 | 0.77 | 0.49 |
| External validation set | 427 | 0.53 | 0.47 |
A lower R2 value was obtained for external validation set because this set had a much narrower variation range of both log D, and log Pe values. Nevertheless, consistently low RMSE values indicate that the model is not-overfitted and can predict passive permeability close to the level of experimental uncertainty.
Reference database
In addition to HIA and Caco-2 permeability predictions, ACD/Percepta provides Absorption DB - a a browsable database of human intestinal absorption & bioavailability. The experimental data were compiled from reference pharmacokinetic tabulations and original articles, the main sources being "Therapeutic Drugs" (ed. by C. Dollery), Goodman & Gilman's "The Pharmacological Basis of Therapeutics", and a compilation by Zhao, Y. H. et al. J Pharm Sci. 2001;90(6):749-84. [7] A qualitative assignment of absorption category (good, moderate, poor) is provided for each compound in the database along with comments regarding the quantitative extent of absorption/bioavailability, presence of carrier-mediated transport, etc. where available.
Transport mechanisms
The predictive models comprising the Passive Absorption module account for passive transport of analyzed compounds across intestinal barrier. In order to propose a clear physicochemical explanation of passive diffusion process, the data used for model development were thoroughly evaluated to exclude values affected by enzymatic efflux or influx. For such compounds special alerts are displayed in the Oral Bioavailability module indicating that expected oral bioavailability/absorption values for these molecules may be higher or lower due to presence of carrier-mediated processes.